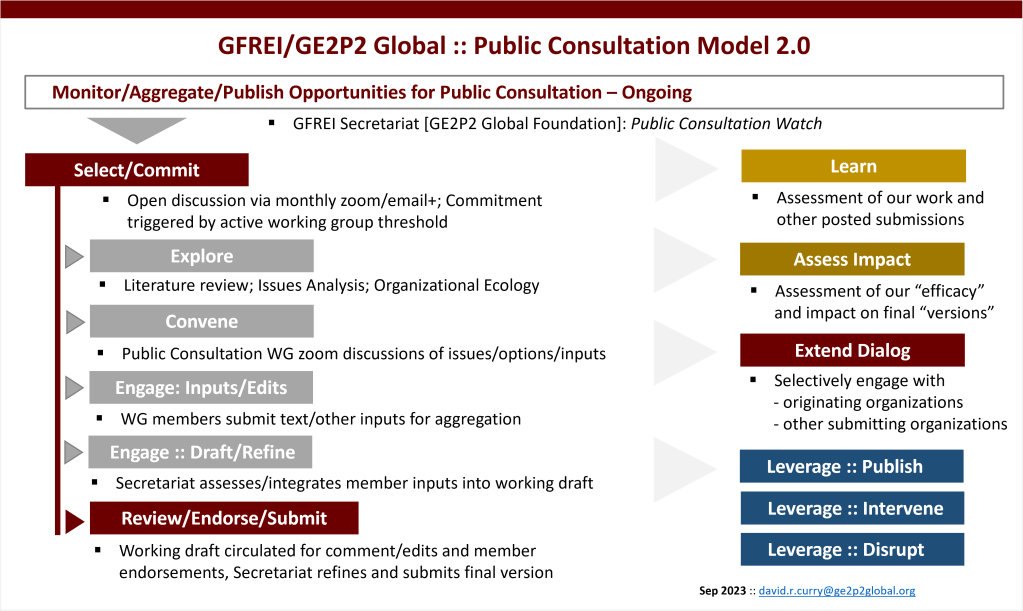
We are building an inventory of our public consultation submissions and other publications from the last several years for reference.
We continue to refine our model for developing public consultation responses and invite comment and inquiries:
.::::::::::::
GE2P2 Global Foundation :: Global Consultation Submission – Draft UNESCO Recommendation on Ethics of Neurotechnology – 12 July 2024
Neurotechnology is a fast-expanding field dedicated to understanding the brain and creating technologies that interact with it. Since neurotechnology can offer promises to address mental health issues, along with so called “wellbeing”, neuromarketing, neurogaming. Shouldn’t we be thinking about how women and men will be included in the development of these technologies to harness the potential benefits for all? What can be the role of stereotypes in targeting men and women in the applications that are not related to health?
Towards an International Instrument
From June to mid-July 2024, global, regional, sub-regional and national consultations will be conducted by UNESCO in order to collect the views of a wide range of key stakeholders and to incorporate pluralistic perspectives into the first draft of the Recommendation, ensuring an open and inclusive elaboration process. Join the Global Consultation by responding to the Online Questionnaire on the first draft of the Recommendation on the Ethics of Neurotechnology. Deadline: 12 July 2024
::::::::::::
Global Forum for Research Ethics and Integrity [GFREI]-GE2P2 Global :: Public Comment on Declaration of Helsinki – 2024 Revision: Phase 2 – 24 June 2024 Summary
The workgroup’s proposed edits to date are now available for public comment. To submit your organization’s or your individual feedback, download the phase 2 public comment document and populate your comments in the row that corresponds to the DoH paragraph under review. If you wish to offer specific edits for consideration, indicate proposed new language with bold yellow highlighted language. Indicate any proposed deletions with a yellow highlighted strikethrough language. Please do not use Track Changes. Please ensure all comments are properly attributed by including your name, title and organisation. Email the completed document to doh@wma.net no later than 24 June 2024, 5pm Central Time (UTC-6). Since this is the final comment period, submissions received after June 24 will not be considered.
Consideration for Adoption: General Assembly Meeting Helsinki, Finland
The workgroup intends to recommend a final updated draft of the Declaration of Helsinki to the Medical Ethics Committee of the World Medical Association, and the document is intended to be considered by the Council and the General Assembly in Helsinki, Finland in October 2024.
[1] The workgroup includes World Medical Association Constituent Members from Bangladesh, Belgium, Brazil, China, Denmark, Finland, Germany, Israel, Italy, Japan, Malaysia, Netherlands, Nigeria, South Africa, Taiwan, United Kingdom, United States (Chair), Uruguay, Vatican and the World Medical Association Associate Members
::::::::::::
GE2P2 Global – GFREI :: Public Comment – Draft WHO Principles for human genome access, use, and sharing – 03 May 2023
For the potential of genomics to be realized, access to, use, and the sharing of human genome data is critical. Following the WHO’s Science Council 2022 Report on Accelerating access to genomics for global health: promotion, implementation, collaboration, and ethical, legal, and social issues, WHO is implementing a programme of activities to promote equitable and fair access to genomics technologies for the benefit of people worldwide.
As part of this, WHO is developing guiding principles for human genome data access, use and sharing. To develop these principles, a virtual consultation was held in January 2024 with an interdisciplinary group of participants. This consultation discussed the diverse perspectives on issues related to human genome data access, use and sharing; how a global set of principles from WHO may enable data access, use and sharing; and proposed initial principles. This was followed by an in-person meeting in March 2024 that considered in detail the proposed principles. Following this meeting, a draft document was developed and comments on this document and the principles are now invited
::::::::::::
GE2P2 Global – GFREI Public Comment :: Key Information and Facilitating Understanding in Informed Consent; Draft Guidance for Sponsors, Investigators, and Institutional Review Boards – 30 April 2024
…This draft guidance provides recommendations related to two provisions of the revised Federal Policy for the Protection of Human Subjects (the revised Common Rule) by the U.S. Department of Health and Human Services (HHS) and identical provisions in FDA’s proposed rule “Protection of Human Subjects and Institutional Review Boards.” FDA’s proposed rule, if finalized, would harmonize certain sections of FDA’s regulations on human subject protections and institutional review boards (IRBs), to the extent practicable and consistent with other statutory provisions, with the revised Common Rule, in accordance with the 21st Century Cures Act (Cures Act). The guidance addresses the provisions of the revised Common Rule that require informed consent to begin with key information about the research and to present information in a way that facilitates understanding and identical provisions in FDA’s proposed rule.
::::::::::::
GFREI Public Consultation Submission :: VolREthics initiative :: DRAFT – Global Ethics Charter for the Protection of Healthy Volunteers in Clinical Trials :L 02 April 2024 : Curry, DR on behalf of GFREI. In this letter, the Global Forum for Research Ethics and Integrity [GFREI] – an open, independent collaborative involving 80+ individuals in 30+ countries – provides an analysis of the draft T« Global Ethics Charter for the Protection of Healthy Volunteers in Clinical Trials ». The VolREthics initiative was set up to promote ethical guidelines to protect healthy volunteers in biomedical research, noting that “every year, tens of thousands of people with no known major medical problems participate in research studies aimed at testing the tolerability and behavior within the body of medicines, vaccines, and other biomedical interventions. These people do not derive any direct medical benefit from their participation. The fact that they are healthy helps to avoid any changes in the behavior of the drug related to various diseases. They are called « healthy volunteers » to differentiate them from patients who suffer from the diseases in question, and for whom participation in biomedical research may provide medical benefit. The central ethical question raised by the involvement of healthy volunteers in biomedical research is that of respect for the person’s free will to decide to participate in the research, without any direct or indirect pressure. Another ethical imperative is to ensure the safety and well-being of the healthy volunteers both during and after the research. This is because all biomedical research involves a certain number of risks that, even if kept to a minimum by the researchers and authorities, can never be ruled out entirely.. VolREthics is supported by the Drugs for Neglected Diseases initiative (DNDi), as well as for certain events by partners such as the Council for International Organisations of Medical Sciences (CIOMS), Council of Europe, European Network of Research Ethics Committees (EUREC), European Commission, World Health Organization, European and Developing Countries Clinical Trials Partnership (EDCTP), UNESCO, and Wellcome Trust.
::::::::::::
Strengthening global health policies, guidance, regulations: responding to calls for public consultation by integrating diverse voices, experience and expertise
Wellcome Open Research, Open Letter, 10 Jan 2024 [version 1; peer review: awaiting peer review]
David R Curry, Sedem Adiabu, Daima Bukini, Lynn Ang, Thalia Arawi, Beate Aurich, Mariam Hassan, Fernando Lolas, Rita S Sitorus, Yeyang Su
Abstract
In this open letter, members of the Global Forum for Research Ethics and Integrity [GFREI] – an open, independent collaborative involving 80+ individuals in 30+ countries – report on and extend arguments made during a workshop conducted at the Oxford Global Health and Bioethics International Conference (June 26–27, 2023). Ten GFREI presenters from nine countries used a case study approach to present and discuss an emerging model to integrate diverse global voices in engaging open public consultation opportunities – sometimes termed calls for input, calls for public comment, requests for information (RFIs). A key form of public consultation invites observations and ideas on, and specific edits to, draft versions of policies, guidance, regulations, and other formats under a specific deadline. Based on our experience, we posit that engagement of public consultation opportunities is a matter of transnational civic responsibility; that such engagement can add substantive value to public discourse and the realization of public good; that engaging voices and views from all global regions, and respecting and integrating diverse contributions from varying experience, expertise and forms of knowledge significantly strengthens public consultation responses; and that sustaining and extending GFREI’s independent global voice, and building its diversity and the range of disciplines represented, is worthy of robust support from the global community. Finally, we call on the ethics/bioethics community to engage these calls as an important strategy in advancing the ethical soundness of the laws, regulations, policies, guidelines presented for public consultation.
::::::::::::
GFREI Public Consultation Submission: ICH E6(R3) GCP Principles, Annex 1 – Draft. (19 May 2023) Curry, D.R., Adiabu, S., Andrade-Narvaez, F.J., Ang, L., Asif, F., Arawi, T., Aurich, B. Bierer, B.E., Collet, M.C., DeTora, L., Diniz, N.M., Gispen-de Wied, C.C., Kang, J.S., Klein, R., Kumar, N., Mahasha, P.W., Pavlov, C.S., Redman, B., Ross, G., Schatz, G.S., Sitorus, R.S., as the Global Forum on Research Ethics and Integrity [GFREI] Working Group/GE2P2 Global Foundation. (05 September 2023)., https://wordpress.com/page/ge2p2-center.net/22763 FDA Posted Version: https://www.regulations.gov/comment/FDA-2023-D-1955-0041
::::::::::::
GFREI RFI Submission: NIH Promoting Equity in Global Health Research, NOT-TW-22-001 – May 20, 2022. Curry, D.R., Adiabu, S., Andrade-Narvaez, F.J., Ang, L., Arawi, T., Asif, F., Aurich, B., Bierer, B.E., Bukini, D.A., Crawley, F.P., Dalla Costa, E., Diniz, N.M., Dobrova, V., Engler-Todd, Freitas, M., Hassan, M. Iceland, K., Ike, C., Kebede, S., Kumar, N., Kurihara, C., Lolas, F., Mboera, L., Muthuswamy, V., Pavlov, C.S., Prichep, E., Ross, G., Sitorus, R.S., Su, Y., Suzuki, M., Ukpong-Folayan, M., Yimer, G. as the Global Forum on Research Ethics and Integrity [GFREI] Working Group/GE2P2 Global Foundation. 03 August 2022. https://wordpress.com/page/ge2p2-center.net/22778
::::::::::::
GFREI Public Consultation Submission: WHO Guidance for Global Practices for Clinical Trials – Draft. (19 July 2023). Curry, D.R., Adiabu, S., MPH, Doe Anderson, J., Andrade-Narvaez, F.J., Ang, L., Arawi, T., Aurich, B., DeTora, L., Diniz, N., Gispen-de Wied, C.C., Klein, R., Mahasha, P.W., Pavlov, C.S., Redman, B., Schatz, G.S., Sitorus, R.S., Suzuki, M. as the Global Forum on Research Ethics and Integrity [GFREI] Working Group/GE2P2 Global Foundation. (22 September 2023). https://wordpress.com/page/ge2p2-center.net/22742
::::::::::::